HARD
Earn 100
An ammonia -ammonium chloride buffer has a value of with . What will be the new if is added to buffer solution .
(a)
(b)
(c)
(d)
66.67% studentsanswered this correctly
Important Questions on Ionic Equilibrium
EASY
MEDIUM
EASY
EASY
HARD
[Given: of acetic acid molar mass of acetic acid , Neglect any changes in volume]
MEDIUM
[Given:
MEDIUM
Which of the following mixtures will have the lowest pH at
MEDIUM
MEDIUM
HARD
A solution of weak base is titrated with of a strong acid . The variation of of the solution with the volume of added is shown in the figure below. What is the of the base? The neutralisation reaction is given by .
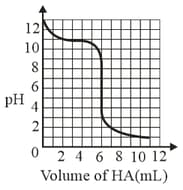
MEDIUM
MEDIUM
HARD
HARD
MEDIUM
EASY
EASY
HARD
EASY

