MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Earn 100
An athlete is given of glucose of energy equivalent to . He utilises percent of this gained energy in the event. In order to avoid storage of energy in the body, determine the weight of water he would need to perspire. (The enthalpy of evaporation of water is ).

Important Questions on Thermodynamics & Thermochemistry
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
From the following data, determine the enthalpy change for the sublimation of ice at . [Mean heat capacity of ice, mean heat capacity of , mean heat capacity of , enthalpy of fusion of ice at , enthalpy of evaporation of water at ]
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
The heat of formation of is . The heat of combustion of is for and are and respectively. Then determine the for the isomerisation reaction , and for the same are at .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
In the reaction, of , the enthalpies of formation of are in the ratio of and have opposite sign. Determine the value of .
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
and are diatomic molecules. If the bond enthalpies of are in the ratio and enthalpy of formation of from and is . What is the bond enthalpy of ?
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
When a certain amount of ethylene was combusted, heat was evolved. If heat of combustion of ethylene is, determine the volume of (at ) that entered into the reaction.
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Given the following reactions:
I:
II:
Which amongst and is more stable?
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Enthalpy of polymerisation of ethylene, as represented by the reaction, is per mole of ethylene. Given bond enthalpy of bond is , determine enthalpy of bond (in ).
MEDIUM
KVPY Aptitude Test - Stream SA
IMPORTANT
Substance can undergo decomposition to form two sets of products:
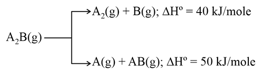
If the molar ratio of to is in a set of product gases, determine then the energy involved in the decomposition of mole of .
