HARD
Earn 100
An earthen pitcher loses of water per minute due to evaporation. If the water equivalent of pitcher is and the pitcher contains of water, calculate the time required for the water in the pitcher to cool to from its original temperature of . Neglect radiation effects. Latent heat of vapourization of water in this range of temperature is and specific heat of water is .
(a)
(b)
(c)
(d)
41.33% studentsanswered this correctly
Important Questions on Thermal Properties of Matter
MEDIUM
[Take specific heat of water and latent heat of steam
EASY
Heat required to melt of ice is . A man melts of ice by chewing in one minute. His power is______
EASY
EASY
EASY
MEDIUM
EASY
A steam engine intakes of steam at per minute and cools it down to . If latent heat of vaporization of steam is , then the heat rejected by the steam engine per minute is _____
(Given : specific heat capacity of water : )
MEDIUM
EASY
HARD
A thermally insulated cubical box of side length and wall thickness containing of ice is closed on all sides. The mass of ice melted in hours is (Thermal conductivity of the material of the box latent heat of ice and ambient temperature )
EASY
MEDIUM
EASY
EASY
HARD
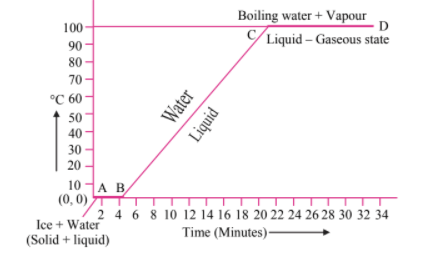
Explain the following temperature versus time graph:
MEDIUM
HARD
(Specific heat of water is and the density of water is )
HARD
MEDIUM
[ Specific heat of water Latent heat of water ]
EASY
(Latent heat of ice is and )

