MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
An electron collides with a fixed hydrogen atom in its ground state. Hydrogen atom gets excited and the colliding electron loses all its kinetic energy. Consequently, the hydrogen atom may emit a photon corresponding to the largest wavelength of the Balmer series. The kinetic energy of the colliding electron is given by . Find the value of .
(a)
(b)
(c)
(d)none of these
100% studentsanswered this correctly

Important Questions on Atomic Physics
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
In the characteristic -ray spectra given in the figure of some atom superimposed on continuous -ray spectra
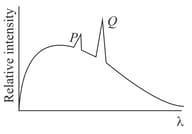
HARD
JEE Main/Advance
IMPORTANT
Statement- : The Bohr model of the hydrogen atom does not explain the fine structure of spectral lines.
and
Statement-: The Bohr model does not take into account the spin of the electron
