EASY
Earn 100
An electron in its orbit undergoes transitions across the energy levels either by absorbing or emitting the photons. A given hydrogen atom is in third excited state.
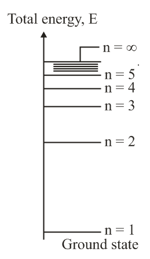
Determine the final quantum number and the energy of the photon,
i. when a photon with shortest wavelength is emitted
ii. when a photon with longest wavelength is absorbed

i. when a photon with shortest wavelength is emitted
Important Questions on Structure of Atoms and Nuclei
MEDIUM
MEDIUM
HARD
[Use and are Planck's constant and speed of light, respectively]
MEDIUM
(Given: )
EASY
EASY
HARD
MEDIUM
MEDIUM
HARD
MEDIUM
(for a photon of wavelength , energy )
MEDIUM
MEDIUM
MEDIUM
HARD
MEDIUM
HARD
MEDIUM
EASY
MEDIUM

