An electron moving from left to right meets an obstacle, which in one case is a step (Figure (a)), and in the other a barrier (Figure (b)). What are the probabilities of the electron overcoming the step and the barrier according to the classical theory and the quantum theory in two separate cases, namely, when the electron kinetic energy is lower than and when it is higher than
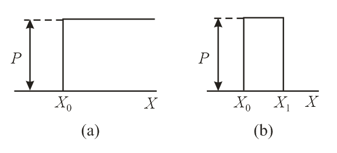
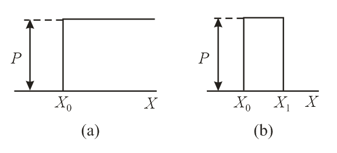

Important Questions on Atomic and Nuclear Physics
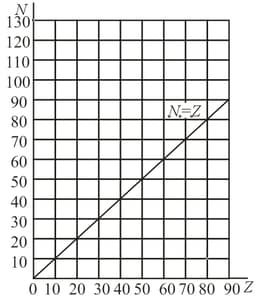
The number of protons and the number of neutrons in the nuclei of stable isotopes are laid off on the horizontal and vertical axes, respectively. Why does the fraction of neutrons in the overall number of nucleons increase with the mass number of the nuclei?
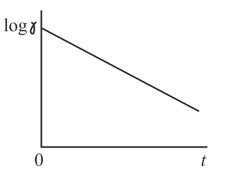
A counter registers the rate of radioactive decay, that is, the number of radioactive decay acts taking place every second. The results obtained in such measurements are plotted in the form of a diagram in which the time interval from the beginning of counting is laid off on the horizontal axis and the logarithm of the decay rate, on the vertical axis. How to find the half-life of the radioactive element from such a diagram?
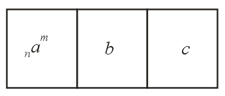
In the Periodic Table, we select three consecutive elements, say, , and . A radioactive isotope of element whose proton and mass numbers are placed at the symbol of the element transforms into element , which in turn transforms into element . This last element transforms into an isotope of the initial element . What processes cause the transformations and ? What are the proton and mass numbers of the nuclei of elements and and those of the nucleus of element after the final transformation is completed?
In beta decay, the velocity of the nucleus that emits an electron is not directed along the line along which the electron velocity is directed. How can this phenomenon be explained?

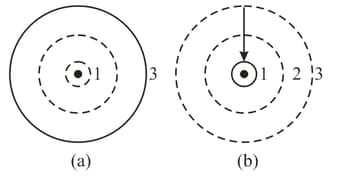
Within the framework of the "classical" Bohr theory, an excited atom is an atom one electron of which moves along an orbit that is farther from the nucleus than in the ground state (Figure (a)). When the atom goes over to its ground state (Figure (b)), the atom emits a photon. In the literature, especially popular-science literature, the common way to describe this process is to say that mass has transformed into energy. Is this' actually the case?