An element forms two oxides and . The oxide is neutral but is acidic in nature. The element is most likely to be
Important Questions on Metals and Non-Metals
An element X (atomic number 20) reacts with another element Y (atomic number 17) to form a compound X. Which of the following statement are true regarding compound Z. Which of the following statement are true regarding this compound?
I. Molecular formula of of X is .
II. X and Y are joined by sharing of electrons.
III. Z imparts characteristic flame colour.
IV. It is soluble in water.
An element forms two oxides and . The oxide is neutral but is acidic in nature.The element is most likely to be
Four elements W, X, Y, and Z have atomic numbers 12,18, 4, and 16:
Which of the above will form an acidic oxide?
What is the product when sulphur reacts with oxygen?
Which of the following products are obtained when hydrogen reacts with chlorine atom?
Observe the given figure carefully.
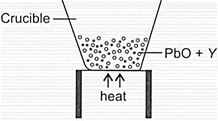
The residue left behind in the crucible, substance and the substance which can replace in the above process are respectively
Nitric acid oxidises sulphur to:

