An element from group of the periodic table reacts with an element from group to form a compound.
What is the valency of element?

Important Questions on Periodic Classification Of Elements
The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters and (which are not their chemical symbols):
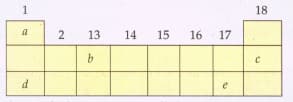
Select the letter which represents an alkali metal.
The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters and (which are not their chemical symbols):
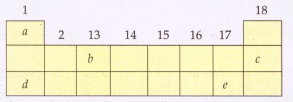
Select the letter which represents a noble gas.
The following diagram shows a part of the periodic table containing first three periods in which five elements have been represented by the letters and (which are not their chemical symbols):
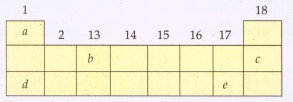
Select the letter which represents a halogen.
Which two elements will form a covalent compound?
Which two elements will form an ionic compound?
To which group or period of the periodic table do these elements belong?
What would be the nature of compound formed by a combination of elements and ?
