EASY
Earn 100
An experiment is performed to measure the specific heat of copper. A lump of copper is heated in an oven, then dropped into a beaker of water. To calculate the specific heat of copper, the experimenter must know or measure the value of all quantities below except the
(a)heat capacity of water and breaker
(b)original temperature of the copper and the water
(c)final (equilibrium) temperature of the copper and the water
(d)time taken to achieve equilibrium after the copper is dropped into the water
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
HARD
MEDIUM
MEDIUM
What will be the molar specific heat at constant volume of an ideal gas consisting of rigid diatomic molecules?
EASY
EASY
HARD
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
HARD
EASY
EASY
EASY
MEDIUM
Two different metal bodies and of equal mass are heated at a uniform rate under similar conditions. The variation of temperature of the bodies is graphically represented as shown in the figure. The ratio of specific heat capacities is:
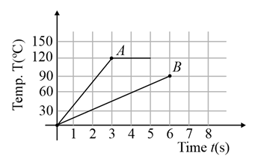
MEDIUM
[Given that
EASY
EASY
(Take gas constant )

