An ice cube is heated and the variation of its temperature with time is shown. The process representing the conversion of water into steam is
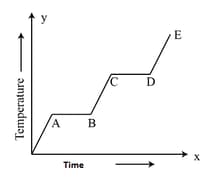

Important Questions on Thermal Properties of Matter
(Latent heat of ice is and )
A thermally insulated cubical box of side length and wall thickness containing of ice is closed on all sides. The mass of ice melted in hours is (Thermal conductivity of the material of the box latent heat of ice and ambient temperature )
A steam engine intakes of steam at per minute and cools it down to . If latent heat of vaporization of steam is , then the heat rejected by the steam engine per minute is _____
(Given : specific heat capacity of water : )
[ Specific heat of water Latent heat of water ]
[Take specific heat of water and latent heat of steam
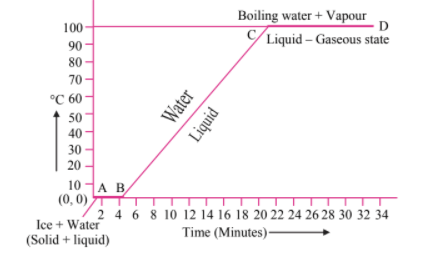
Explain the following temperature versus time graph:
Heat required to melt of ice is . A man melts of ice by chewing in one minute. His power is______

