An ice cube of mass at is left sitting on the kitchen table where it gradually melts and reaches the kitchen temperature of . The latent heat of fusion for ice is and the specific heat of water is . Select the correct statement(s) about this process. (All numerical values in the answers are rounded to the closest integer.)
Important Questions on Thermodynamics
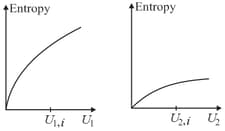
Graphs below show the entropy versus energy of two systems and at constant volume. The initial energies of the systems are indicated by and respectively. Graphs are drawn to the same scale. The systems are then brought into thermal contact with each other. Assume that, at all times the combined energy of the two systems remains constant. Choose the most appropriate option indicating the energies of the two systems and the total entropy after they achieve the equilibrium.
Step It is first compressed adiabatically from volume to
Step then expanded isothermally to volume
Step then expanded adiabatically to volume
Step then compressed isothermally to volume If the efficiency of the above cycle is then is
Given the following entropy values at 298 K and 1 atm : The entropy change for the reaction
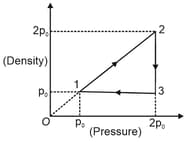
The density versus pressure graph of one mole of an ideal monoatomic gas undergoing a cyclic process is shown in the figure. The molecular mass of gas is .
Find work done in each process.
Find heat rejected by gas in one complete cycle.
Find the efficiency of the cycle.
Represent the union of two sets by Venn diagram for each of the following.
is a prime number between and
is an odd number between and