EASY
Earn 100
An ideal diatomic gas is taken through a process as shown in the diagram. The molar heat capacity of the gas in this process will be:
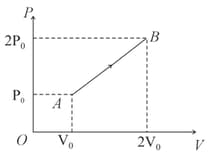

(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
What will be the molar specific heat at constant volume of an ideal gas consisting of rigid diatomic molecules?
MEDIUM
EASY
EASY
EASY
HARD
EASY
EASY
MEDIUM
[Given that
MEDIUM
EASY
EASY
(Take gas constant )
HARD
For a monoatomic ideal gas following the cyclic process shown in the vs plot, identify the incorrect option:

MEDIUM
MEDIUM
Match the following: (where is gas constant)
| Column I | Column II | ||
| (a) | Molar specific heat of helium gas at constant volume | (i) | |
| (b) | Molar specific heat of oxygen at constant volume | (ii) | |
| (c) | Molar specific heat of carbon dioxide at constant volume | (iii) | |
| (d) | Molar specific heat of hydrogen at constant pressure | (iv) |
EASY
MEDIUM
An ideal gas undergoes a four step cycle as shown in the diagram below. During this cycle, heat is absorbed by the gas in:
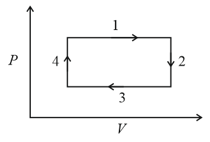
EASY
EASY
MEDIUM

