HARD
Physics
IMPORTANT
Earn 100
An ideal gas can be expanded from an initial state to a certain volume, through two different processes: constant and , where is a positive constant. Then,
(a)Final temperature in will be greater than that in .
(b)Final temperature in will be greater than that in .
(c)Total heat given to the gas in case is greater than that in .
(d)Total heat given to the gas in case is greater than that in .
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
Physics
IMPORTANT
EASY
Physics
IMPORTANT
MEDIUM
Physics
IMPORTANT
A cyclic process is shown in the P–V diagram. ( and are isothermal)
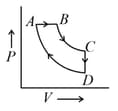
Which of the following curves represent the same process?
MEDIUM
Physics
IMPORTANT
MEDIUM
Physics
IMPORTANT
MEDIUM
Physics
IMPORTANT
EASY
Physics
IMPORTANT
When a system is taken from state to state along the path , it is found that and . Along the path , then work (in ) along the path is-

EASY
Physics
IMPORTANT
