MEDIUM
JEE Main/Advance
IMPORTANT
Earn 100
An ideal gas filled at pressure of atm and temp of in a balloon is kept in vacuum with in a large insulated container. The wall of balloon is punctured then container temperature:
(a)Decreases
(b)Increases
(c)Remain constant
(d)Unpredictable
50% studentsanswered this correctly

Important Questions on States of Matter: Gases and Liquids
HARD
JEE Main/Advance
IMPORTANT
What is the order of partial pressure of water vapours at equilibrium and relative humidity respectively?
HARD
JEE Main/Advance
IMPORTANT
The vapour pressure of water at is . A vessel contained water-saturated oxygen at , the total gas pressure being . The contents of the vessel were pumped into a vessel at the same temperature. What were the partial pressures of oxygen and water vapour, respectively and the total pressure in the final equilibrium state? Neglect the volume of water which might condense.
HARD
JEE Main/Advance
IMPORTANT
A vessel has nitrogen gas and water vapours in equilibrium with liquid water at a total pressure of . The partial pressure of water vapours is . If the volume of this vessel is reduced to one-third of its original volume, at the same temperature, then, find the total pressure of the system. (Neglect the volume occupied by liquid water)
EASY
JEE Main/Advance
IMPORTANT
When a liquid that is immiscible with water was steam distilled at at a total pressure of the distillate contained of the liquid per gram of water. The vapour pressure of water is at what is the molar mass of liquid?
EASY
JEE Main/Advance
IMPORTANT
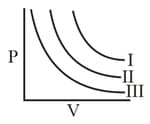
are three isotherms respectively at and as shown in graph. Temperature will be in order:
MEDIUM
JEE Main/Advance
IMPORTANT
A of a mixture of and at and pressure was sparked so that the formation of water was complete. The remaining pure gas had a volume of at and pressure. If the remaining gas was , the mole fraction of in the mixture is:
HARD
JEE Main/Advance
IMPORTANT
On the surface of the earth at pressure, a balloon filled with gas occupies . This volume is of its maximum stretching capacity. The balloon released left in the air. It starts rising. Calculate the height above which the balloon will burst if the temperature of the atmosphere remains constant and the pressure decreases by for every rise in the height. (nearest integer)
MEDIUM
JEE Main/Advance
IMPORTANT
A vessel of volume litre contains of nitrogen at a temperature . The pressure of the gas is of its molecules are dissociated into atoms at this temperature is:
