MEDIUM
JEE Main
IMPORTANT
Earn 100
An ideal gas goes through a reversible cycle has the V - T diagram shown below. Process are adiabatic.
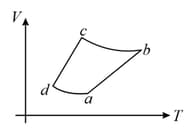
The corresponding P - V diagram for the process is (all figures are schematic and not drawn to scale) :
(a)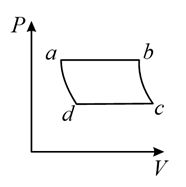

(b)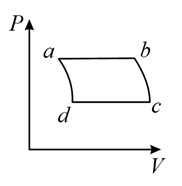

(c)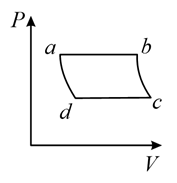

(d)

50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Main
IMPORTANT
Consider a spherical shell of radius R at temperature T. The black body radiation inside it can be considered as an ideal gas of photons with internal energy per unit volume and pressure . If the shell now undergoes an adiabatic expansion the relation between T and R is:
HARD
JEE Main
IMPORTANT
A solid body of constant heat capacity is being heated by keeping it in contact with reservoirs in two ways: (i) Sequentially keeping in contact with 2 reservoirs such that each reservoir supplies the same amount of heat. (ii) Sequentially keeping in contact with reservoirs such that each reservoir supplies the same amount of heat. In both, cases the body is brought from initial temperature to final temperature . Entropy change of the body in the two cases respectively is: Note: This question was awarded as a bonus since temperatures were given in centigrade instead of in Kelvin. Proper corrections are made in the question to avoid it.
MEDIUM
JEE Main
IMPORTANT
A monoatomic gas is compressed from a volume of to a volume of at a constant pressure of . Then it is heated at constant volume by supplying of energy. As a result, the internal energy of the gas
MEDIUM
JEE Main
IMPORTANT
The equation of state for a gas is given by , where n is the number of moles and is a positive constant. The initial temperature and pressure of one mole of the gas contained in a cylinder are T0 and P0 respectively. The work done by the gas when its temperature doubles isobarically will be :
MEDIUM
JEE Main
IMPORTANT
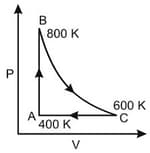
One mole of diatomic ideal gas undergoes a cyclic process ABC as shown in figure. The process BC is adiabatic. The temperatures at A, B and C are 400 K, 800 K and 600 K respectively. Choose the correct statement :
HARD
JEE Main
IMPORTANT

The above diagram represents the thermodynamic cycle of an engine, operating with an ideal mono-atomic gas. The amount of heat, extracted from the source in a single cycle, is:
EASY
JEE Main
IMPORTANT
A Carnot engine whose heat sinks is at , has an efficiency of . By how many degrees should the temperature of the source be changed to increase the efficiency by of the original efficiency ?
MEDIUM
JEE Main
IMPORTANT
A Carnot engine takes of heat from a reservoir at and gives heat to a sink at . The work done by the engine is
