MEDIUM
JEE Advanced
IMPORTANT
Earn 100
An ideal gas in a thermally insulated vessel at internal pressure = , volume = and absolute temperature = expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are , and , respectively. For this expansion,
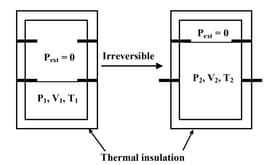
(a)
(b)
(c)
(d)
28.57% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Advanced
IMPORTANT
The standard enthalpies of formation of and glucose (s) at are , and , respectively. The standard enthalpy combustion per gram of glucose at is
( for Glucose)
