EASY
NEET
IMPORTANT
Earn 100
An ideal gas is compressed to half of its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
(a)Isobaric
(b)Isochoric
(c)Isothermal
(d)Adiabatic
35.29% studentsanswered this correctly

Questions featured in Previous Year Papers on Heat & Thermodynamics
MEDIUM
NEET
IMPORTANT
Match Column - I and Column - II and choose the correct match from the given choices.
| Column - I | Column - II |
| (A) Root mean square speed of gas molecules | |
| (B) Pressure exerted by ideal gas | |
| (C) Average kinetic energy of a molecule | |
| (D) Total internal energy of of a diatomic gas |
MEDIUM
NEET
IMPORTANT
HARD
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
EASY
NEET
IMPORTANT
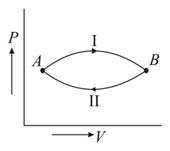
A gas at state changes to state through path and shown in figure. The change in internal energy is and , respectively. Then
HARD
NEET
IMPORTANT
HARD
NEET
IMPORTANT
