MEDIUM
JEE Advanced
IMPORTANT
Earn 100
An ideal gas is expanded from under different conditions. The correct statement(s) among the following is(are)
(a)The work done on the gas is maximum when it is compressed irreversibly from ( to against constant pressure
(b)If the expansion is carried out freely, it is simultaneously both isothermal as well as adiabatic
(c)The work done on the gas is less when it is expanded reversibly from under adiabatic conditions as compared to that when expanded reversibly from under isothermal conditions.
(d)The change in internal energy of the gas (i) zero, if it is expanded reversibly with , and (ii) positive, if it is expanded reversibly under adiabatic conditions with
33.33% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Advanced
IMPORTANT
One mole of an ideal gas at in thermal contact with its surroundings expands isothermally from to against a constant pressure of . In this process, the change in entropy of the surroundings in is:
MEDIUM
JEE Advanced
IMPORTANT
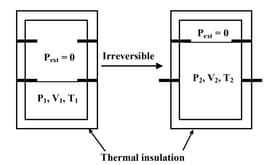
An ideal gas in a thermally insulated vessel at internal pressure = , volume = and absolute temperature = expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are , and , respectively. For this expansion,
HARD
JEE Advanced
IMPORTANT
The standard enthalpies of formation of and glucose (s) at are , and , respectively. The standard enthalpy combustion per gram of glucose at is
( for Glucose)
