MEDIUM
Physics
IMPORTANT
Earn 100
An ideal gas is taken in a cylinder at pressure , volume and temperature . It is taken an isochoric process till its pressure becomes . It is now isothermally expanded to get the original pressure. Finally, the gas is isobarically compressed to its original volume . What is the temperature in the isothermal part of the process?

Important Questions on Kinetic Theory of Gases
MEDIUM
Physics
IMPORTANT
An ideal gas is taken in a cylinder at pressure , volume and temperature . It is taken an isochoric process till its pressure becomes . It is now isothermally expanded to get the original pressure. Finally, the gas is isobarically compressed to its original volume . What is the volume at the end of the isothermal part of the process?
EASY
Physics
IMPORTANT
During an experiment, an ideal gas is found to obey an additional law . The gas is initially at a temperature and volume . Find the temperature when it expands to a volume .
MEDIUM
Physics
IMPORTANT
A mixture of hydrogen and oxygen has volume , temperature , pressure and mass . Calculate the masses of hydrogen and oxygen in the mixture.
MEDIUM
Physics
IMPORTANT
A container contains of hydrogen and of oxygen at ATP (pressure at and temperature ). Chemical reaction is induced by passing electric spark in the vessel till one of the gases is consumed. The temperature is brought back to its starting value . Find the pressure in the container.
MEDIUM
Physics
IMPORTANT
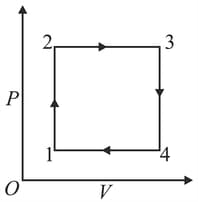
A gas has been subjected to isochoric-isobaric processes (see figure). Plot this process on and diagrams.
MEDIUM
Physics
IMPORTANT
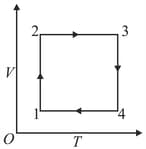
A gas has been subjected to an isothermal-isochoric cycle (see figure). Represent the same cycle on and diagrams.
EASY
Physics
IMPORTANT
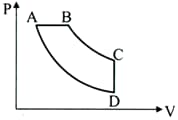
In the pressure-volume diagram given below, the isochoric, isothermal and isobaric parts respectively, are
EASY
Physics
IMPORTANT
An ideal gas expands in such a manner that its pressure and volume can be related by equation constant. During this process, the gas is
