An ideal gas is taken round a cyclic thermodynamic process as shown in the given figure. If the internal energy of the gas at point is assumed zero while at it is . The heat absorbed by the gas in the process is .
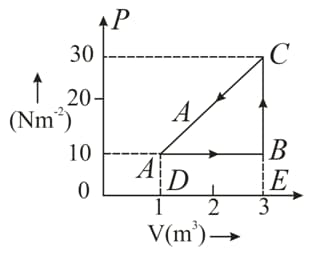
Find the heat energy rejected or absorbed by the gas in the process .
(d) What is the net work done by the gas in the complete cycle $A B C A ?$

(d) What is the net work done by the gas in the complete cycle $A B C A ?$

Important Questions on Thermodynamics
An ideal gas is taken round a cyclic thermodynamic process as shown in the given figure. If the internal energy of the gas at point is assumed zero while at it is . The heat absorbed by the gas in the process is .
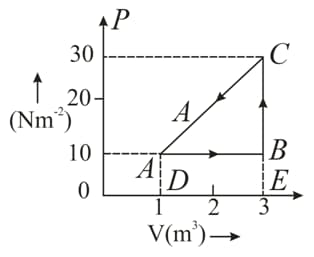
What is the net work done by the gas in the complete cycle
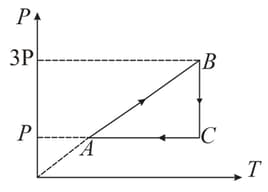
A fixed mass of oxygen gas performs a cyclic process as shown. Find the efficiency of the process.
In moles of a diatomic gas has undergone a cyclic process as shown in figure. Temperature at a is .
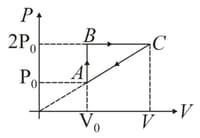
Find volume at .
In moles of a diatomic gas has undergone a cyclic process as shown in figure. Temperature at a is .
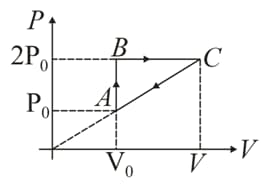
Find maximum temperature.
In moles of a diatomic gas has undergone a cyclic process as shown in figure. Temperature at a is .
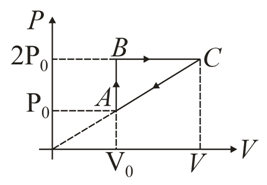
Find total heat given to gas.
In moles of a diatomic gas has undergone a cyclic process as shown in figure. Temperature at a is .
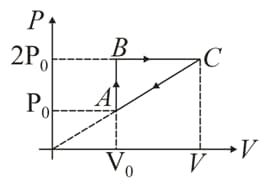
Is heat rejected by the gas. If yes, how much heat is rejected?
In moles of a diatomic gas has undergone a cyclic process as shown in figure. Temperature at a is .
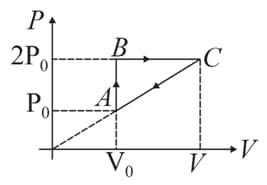
Find out the efficiency.
