HARD
Physics
IMPORTANT
Earn 100
An ideal gas undergoes a cyclic process which is shown by pressure-density curve.
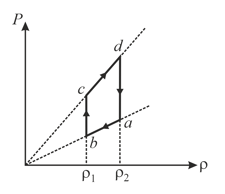

(a)Work done by the gas in the process is zero
(b)Work done by the gas in the process is negative
(c)Internal energy of the gas at point is greater than at state
(d)Net work done by the gas in the cycle is negative.
50% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
Physics
IMPORTANT
EASY
Physics
IMPORTANT
EASY
Physics
IMPORTANT
MEDIUM
Physics
IMPORTANT
A cyclic process is shown in the P–V diagram. ( and are isothermal)
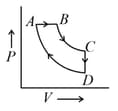
Which of the following curves represent the same process?
MEDIUM
Physics
IMPORTANT
MEDIUM
Physics
IMPORTANT
MEDIUM
Physics
IMPORTANT
EASY
Physics
IMPORTANT
When a system is taken from state to state along the path , it is found that and . Along the path , then work (in ) along the path is-

