An ideal gas undergoes a reversible isothermal expansion at increasing its volume from to . The entropy change of the gas is . How many moles of gas are present?

Important Questions on Entropy and the Second Law of Thermodynamics
Suppose of a monatomic ideal gas is taken from initial pressure and volume , through two steps: an isothermal expansion to volume and a pressure increase to at constant volume. What is for step and step ? What is for step and step ? For the full process, what are and ?
The gas is returned to its initial state and again taken to the same final state but now through these two steps:
an isothermal compression to pressure and a volume increase to at constant pressure. What is for step and step ? What is for
step and step ? For the full process, what are and ?
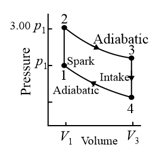
The cycle as shown in the figure represents the operation of a gasoline internal combustion engine. Volume . Assume the gasoline-air intake mixture is an ideal gas with What are the ratios (a), (b) , (c) , (d) and (e) ? (f) What is the engine efficiency?
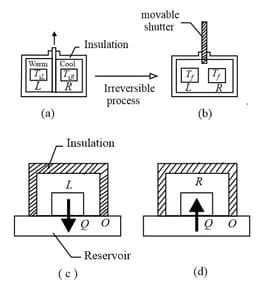
In the irreversible process of figures let the initial temperatures of the identical blocks and be and , respectively, and let be the energy that must be transferred between the blocks in order to reach equilibrium. For the reversible processes of figures , what is (a) for block , (b) its reservoir, (c) block (d) its reservoir, (e) the two-block system, and (f) the system of the two blocks and the two reservoirs?
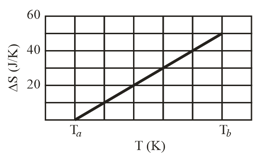
A block is put in contact with a thermal reservoir. The block is initially at a lower temperature than the reservoir. Assume that the consequent transfer of energy as heat from the reservoir to the block is reversible. Figure (as shown below) gives the change in entropy of the block, until thermal equilibrium is reached. The scale of the horizontal axis is set by and . What is the specific heat of the block?