HARD
JEE Advanced
IMPORTANT
Earn 100
An ideal gas undergoes a reversible isothermal expansion from state to state followed by a reversible adiabatic expansion from state to state The correct plot representing the changes from state to state is(are)
(: pressure, : volume, : temperature, : enthalpy, : entropy)
(a)
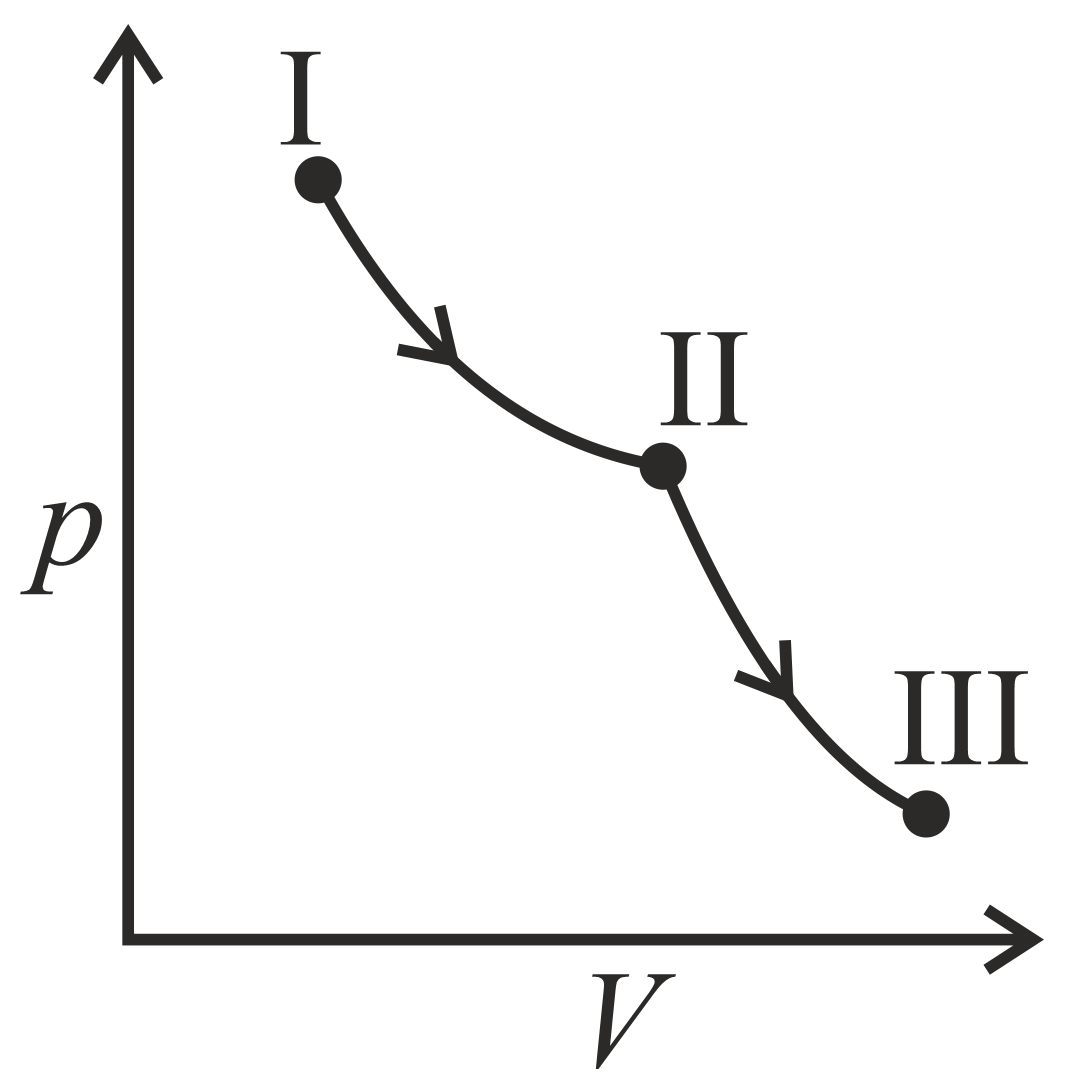
(b)
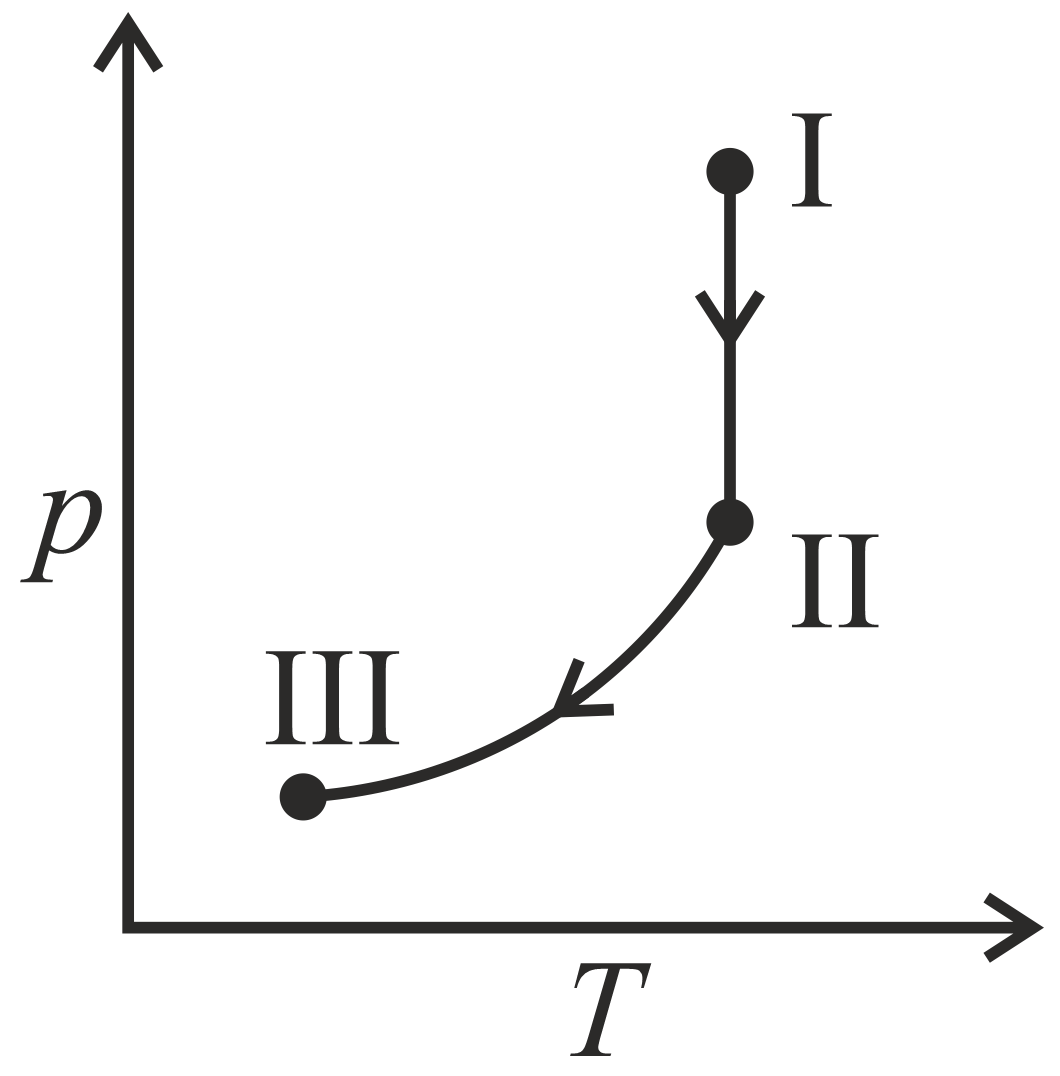
(c)
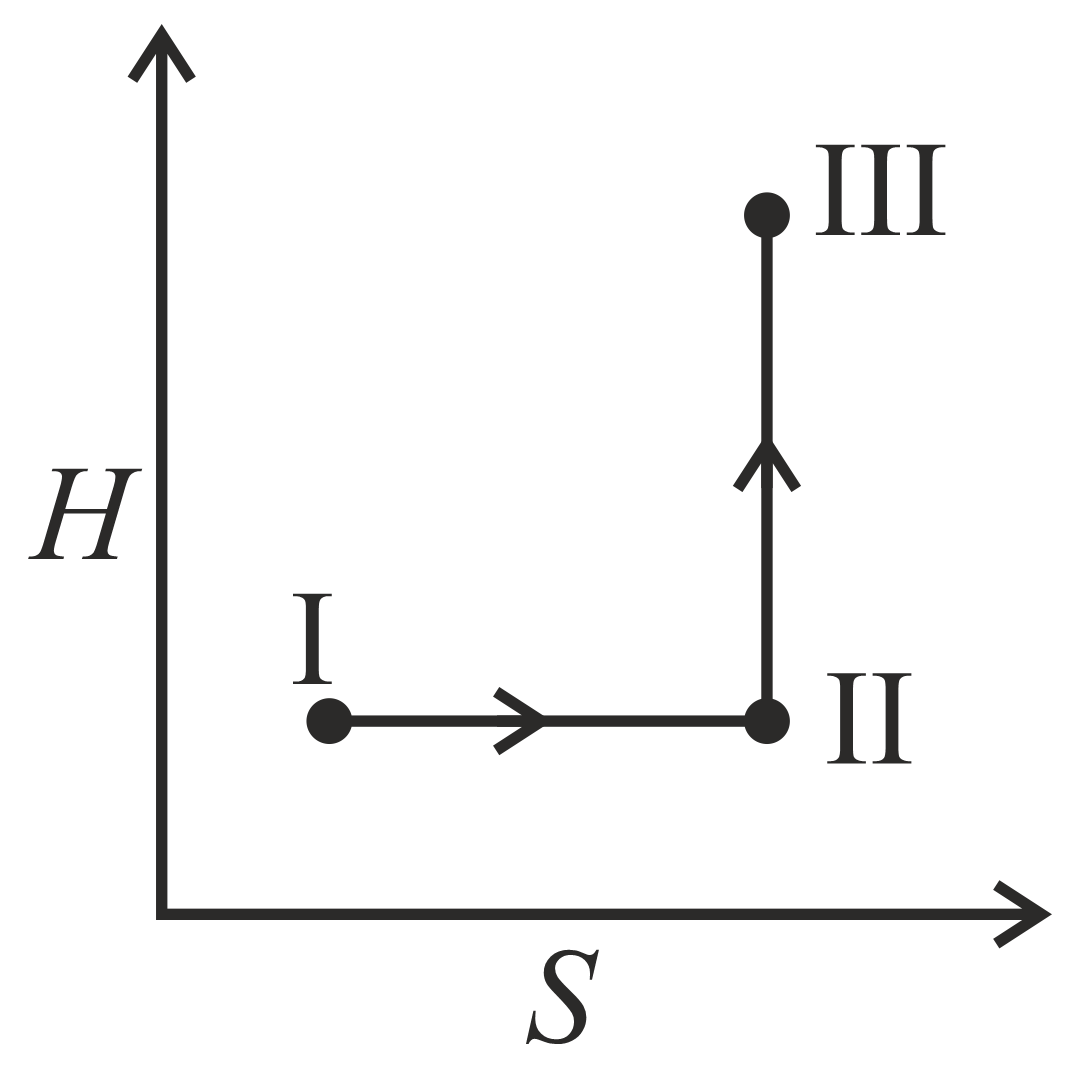
(d)
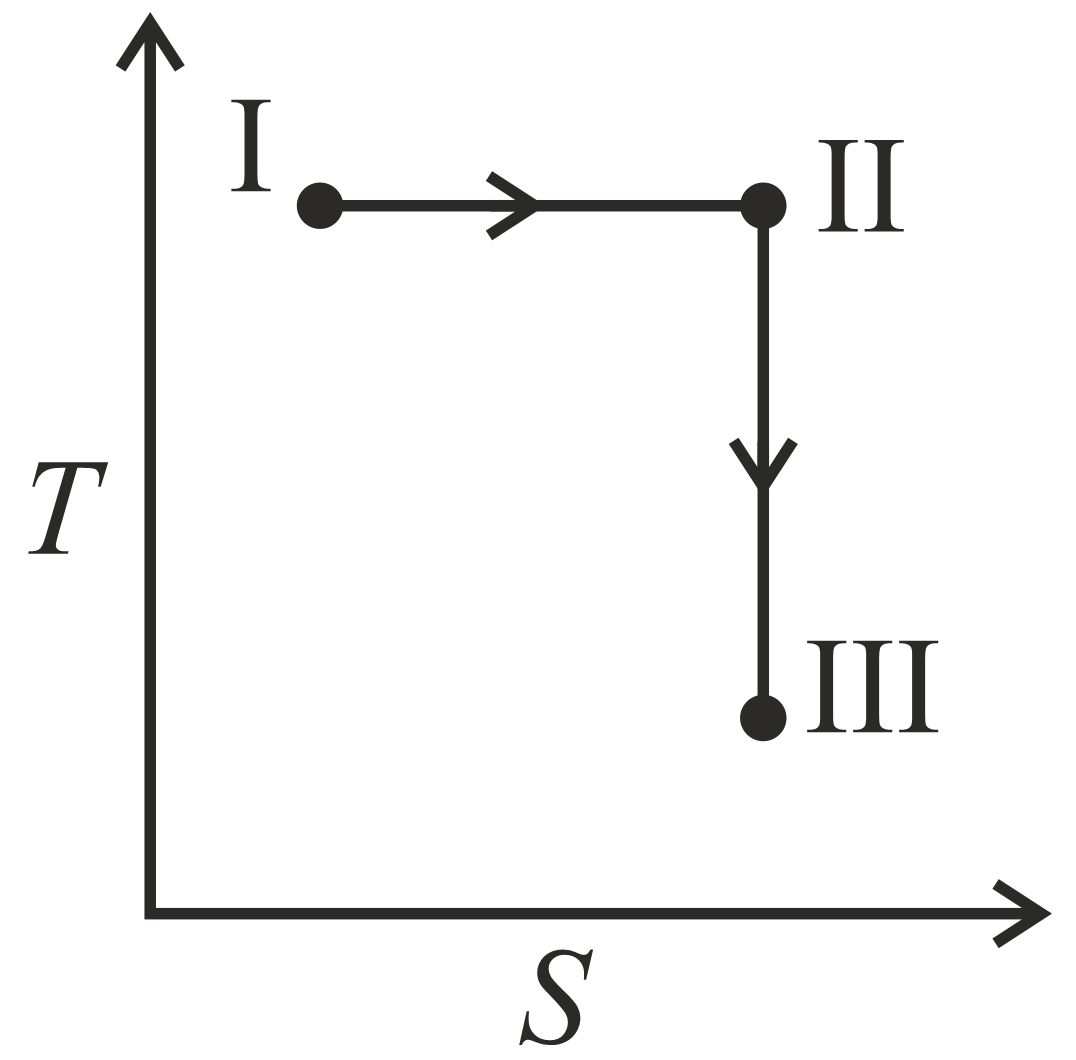
20% studentsanswered this correctly

Important Questions on Thermodynamics
HARD
JEE Advanced
IMPORTANT
Choose the reaction(s) from the following options, for which the standard enthalpy of reaction is equal to the standard enthalpy of formation.
HARD
JEE Advanced
IMPORTANT
A reversible cyclic process for an ideal gas is shown below. Here, are pressure, volume and temperature, respectively. The thermodynamic parameters are heat, work, enthalpy and internal energy, respectively.

The correct option(s) is (are)
HARD
JEE Advanced
IMPORTANT
A closed tank has two compartments A and B, both filled with oxygen (assumed to be ideal gas). The partition separating the two compartments is fixed and is a perfect heat insulator (Figure). If the old partition is replaced by a new partition which can slide and conduct heat but does not allow the gas to leak across (Figure ), the volume (in ) of the compartment A after the system attains equilibrium is . Write the value of .
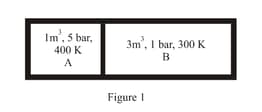
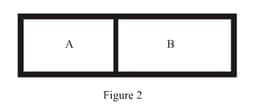
MEDIUM
JEE Advanced
IMPORTANT
An ideal gas is expanded from under different conditions. The correct statement(s) among the following is(are)
HARD
JEE Advanced
IMPORTANT
One mole of an ideal gas at in thermal contact with its surroundings expands isothermally from to against a constant pressure of . In this process, the change in entropy of the surroundings in is:
MEDIUM
JEE Advanced
IMPORTANT
An ideal gas in a thermally insulated vessel at internal pressure = , volume = and absolute temperature = expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are , and , respectively. For this expansion,
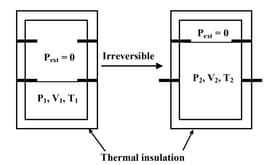
HARD
JEE Advanced
IMPORTANT
The standard enthalpies of formation of and glucose (s) at are , and , respectively. The standard enthalpy combustion per gram of glucose at is
( for Glucose)
