MEDIUM
Chemistry
IMPORTANT
Earn 100
An ideal gas undergoes adiabatic expansion against constant external pressure. Which of the following is incorrect:
(a)Temperature of the system decreases.
(b)The relation constant will be valid (where and are gas variables)
(c)
(d)Enthalpy of the gas remains unchanged.
50% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
Chemistry
IMPORTANT
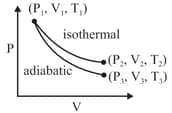
The reversible expansion of an ideal gas under adiabatic and isothermal conditions is shown in the figure. Which of the following statement(s) is (are) correct?
MEDIUM
Chemistry
IMPORTANT
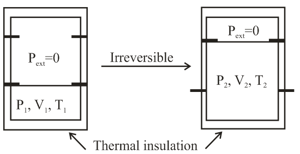
An ideal gas in a thermally insulated vessel at internal pressure volume and absolute temperature expands irreversibly against zero external pressure, as shown in the diagram. The final internal pressure, volume and absolute temperature of the gas are and , respectively. For this expansion
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
HARD
Chemistry
IMPORTANT
MEDIUM
Chemistry
IMPORTANT
In an isothermal expansion of a gaseous sample, the correct relation is: (consider (work) with sign according to new IUPAC convention)
[The reversible and irreversible processes are carried out between same initial and final states.]
HARD
Chemistry
IMPORTANT
