EASY
JEE Main
IMPORTANT
Earn 100
An ideal gas undergoes isothermal expansion at constant pressure. During the process:
(a)Enthalpy remains constant but entropy increases
(b)Enthalpy decreases but entropy increases
(c)Enthalpy increases but entropy decreases
(d)Both enthalpy and entropy remain constant
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
JEE Main
IMPORTANT
A gas undergoes change from state to state . In this process, the heat absorbed and work done by the gas is , respectively. Now gas is brought back to by another process during which of heat is evolved. In this reverse process of .
MEDIUM
JEE Main
IMPORTANT
For the reaction,
and are, respectively, and at 298 K. The equilibrium constant for the reaction at 298 k is:
EASY
JEE Main
IMPORTANT
A reaction at 1 bar is non-spontaneous at low temperature but becomes spontaneous at high temperature. Identify the correct statement about the reaction among the following:
MEDIUM
JEE Main
IMPORTANT
The standard enthalpy of formation of is . If bond enthalpy of is and that of is , the average bond enthalpy of bond in is :
MEDIUM
JEE Main
IMPORTANT
At constant volume, of an ideal gas when heated from to changes its internal energy by The molar heat capacity at constant volume is ________
MEDIUM
JEE Main
IMPORTANT
The magnitude of work done by a gas that undergoes a reversible expansion along the path shown in the figure is _________.
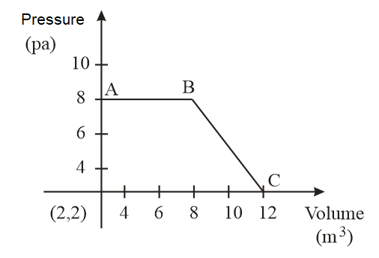
MEDIUM
JEE Main
IMPORTANT
moles of an ideal gas at are allowed to undergo reversible compression till its temperature becomes If calculate and for the process.
MEDIUM
JEE Main
IMPORTANT
For silver, If the temperature of moles of silver is raised from pressure, the value of will be close to:
