An ideal gas with adiabatic exponent is heated at constant pressure. It absorbs amount of heat. Determine the fractions of heat absorbed in raising the internal energy and performing the work.

Important Questions on Thermodynamics
One mole of an ideal monatomic gas undergoes the process , where is a constant.
Find the work done by the gas if its temperature increases by .
One mole of an ideal monatomic gas undergoes the process , where is a constant.
Also, find the molar specific heat of the gas.
An ideal gas is kept in closed vessel of volume , at a temperature of and a pressure of . An amount of joule of heat energy is supplied to the gas. Calculate the final temperature and pressure of the gas.
(Given: The specific heat at constant pressure and gas constant )
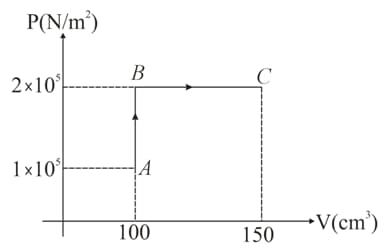
A monoatomic ideal gas is taken in a cylinder. It undergoes through the process as shown in figure. If the temperature at the point is Find
(i) the temperature at points and .
(Use gas constant )
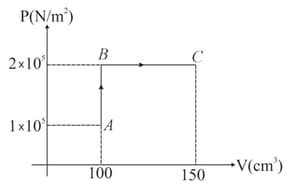
A monoatomic ideal gas is taken in a cylinder. It undergoes through the process as shown in figure. If the temperature at the point is Find
the work done and heat supplied to the gas in paths and
(Use gas constant )