MEDIUM
11th ICSE
IMPORTANT
Earn 100
An ideal monoatomic gas at is compressed adiabatically to th of its original volume. The rise in temperature is
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Isothermal and Adiabatic Processes
MEDIUM
11th ICSE
IMPORTANT
For an ideal gas at constant temperature the correct graph showing variation of with is
MEDIUM
11th ICSE
IMPORTANT
Two samples and of a gas which are initially at the same temperature and pressure, are compressed from volume to ( isothermally and adiabatically). The final pressure of:
MEDIUM
11th ICSE
IMPORTANT
In isobaric, isothermal and adiabatic processes, for the same change in volume, work done is minimum in :
EASY
11th ICSE
IMPORTANT
Which of the following statements is true for a thermodynamic system?
EASY
11th ICSE
IMPORTANT
During the adiabatic expansion of of a gas, the change in internal energy was found to be equal to . The work done during the process will be equal to:
MEDIUM
11th ICSE
IMPORTANT
litre of helium gas at STP is adiabatically compressed to litre. Taking the initial temperature to be , the work done in the process is:
MEDIUM
11th ICSE
IMPORTANT
One kg of a diatomic gas is at a pressure of . The density of the gas is . What is the energy of the gas due to its thermal motion?
MEDIUM
11th ICSE
IMPORTANT
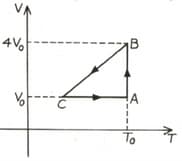
One mole of an ideal gas in initial state undergoes a cyclic process , as shown in the figure. Its pressure at A is . Choose the correct options (s) from the following: