MEDIUM
Earn 100
An idealized diesel engine operates in a cycle known as air standard diesel cycle as shown. Fuel is sprayed into the cylinder at the the point of maximum compression . Combustion occurs during expansion .
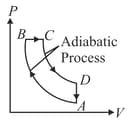

(a)Heat is absorbed during process
(b)Heat is released during process
(c)Heat is absorbed during process
(d)Heat is absorbed during process
50% studentsanswered this correctly
Important Questions on Thermodynamics
MEDIUM
HARD
EASY
EASY
An engine operates by taking a monatomic ideal gas through the cycle shown in the figure. The percentage efficiency of the engine is close to
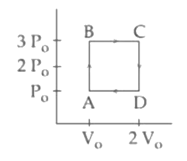
MEDIUM
MEDIUM
EASY
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
Helium gas goes through a cycle (consisting of two isochoric and two isobaric lines) as shown in figure. The efficiency of this cycle is approximately
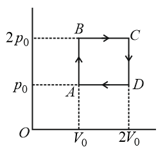
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM

