MEDIUM
CG PET
IMPORTANT
Earn 100
An insulated box containing a diatomic gas of molar mass is moving with a velocity . The box is suddenly stopped. The resulting change in temperature is (Where, is the gas constant)
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Thermodynamics
EASY
CG PET
IMPORTANT
HARD
CG PET
IMPORTANT
mole of gas with is mixed with mole of gas with , then the value of for the resulting mixture is
MEDIUM
CG PET
IMPORTANT
An ideal gas is taken through the cycle , as shown in the figure below. If the net heat supplied to the gas is , then the work done by the gas in the process is
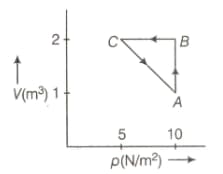
MEDIUM
CG PET
IMPORTANT
HARD
CG PET
IMPORTANT
MEDIUM
CG PET
IMPORTANT
MEDIUM
CG PET
IMPORTANT
