An open-ended mercury manometer is used to measure the pressure exerted by a trapped gas as shown in the figure. Initially, manometer shows no difference in mercury level in both columns as shown in the diagram. After sparking dissociates according to the following reaction
If pressure of Gas decreases to
(Assume temperature to be constant and is )
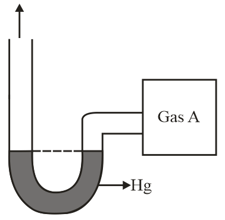
(Assume temperature to be constant and is )

Total pressure increased to

Important Questions on States of Matter: Gases and Liquids
Which of the following is/are correct?
Which of following statement (s) is(are) true
- The slope of isotherm at the critical point is maximum.
-Larger is the value of , easier is the liquification of gas.
-Vander waal's equation of state is applicable below critical temperature at all pressure.
Consider the following statements: If the Van der Waals parameters of two gases are given as:
| Gas X | ||
| Gas Y |
Then (ii)
(iii):
Select the correct statement.
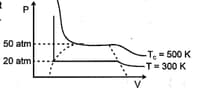
For a real gas, the curve was experimentally plotted, and it had the following appearance. With respect to liquefaction, choose the correct statement: