An organic compound X with the molecular formula, C2H6O on oxidation with potassium dichromate and sulphuric acid, gives an acidic compound Y. The compounds X and Y react on warming in presence of conc. H2SO4 to give a sweet-smelling compound Z. What are the compounds X, Y and Z?
X
Y
Z
(A)
(B)
(C)
(D)

Important Questions on Carbon and its Compounds
Which of the following statements are false about soaps and detergents?
(i) Soaps are water-soluble while detergents are not.
(ii) Soaps are non-biodegradable while detergents are biodegradable.
(iii) Hardness of water is due to the presence of and salts that form scum with soap.
(iv) The polar group in soaps is .
Study the given flow chart carefully and identify W, X and Y.
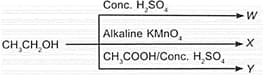
| W | X | Y | |
| (A) | |||
| (B) | |||
| (C) | |||
| (D) |
A few chemical processes are listed as:
I. An alcohol undergoes oxidation to produce a carboxylic acid.
II. An alcohol undergoes esterification.
III. A carboxylic acid reacts with sodium carbonate.
IV. Propane undergoes complete combustion.
In which of the given processes, products have more carbon atoms than the underlined reactant?
In which of the following test tubes, effervescence will be observed?
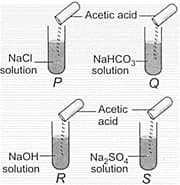
Read the given statements and mark the correct option.
Statement 1: Homologous series is a series of compounds having similar chemical properties.
Statement 2: The successive members of the homologous series differ by group.Match column I with column II and mark the correct option from the given codes:
| Column I | Column II | ||
| (a) | (i) | Conc. | |
| (b) | (ii) | ||
| (c) | (iii) | Alk. | |
| (d) | (iv) | , sunlight |
Methane, the first member of the alkane homologous series has the boiling point equal to . Which of the following represents the correct formula and boiling point of the third member of the series?
| Molecular formula | Boiling Point | |
| (A) | ||
| (B) | ||
| (C) | ||
| (D) |
Two compounds X and Y have the same molecular formula, C3H6O. Identify the functional groups and structural formulae of X and Y.
