An organic compound dissociates into parallel first-order reactions simultaneously and produces different products having rate constants and activation energies , respectively. Calculate the overall energy of activation of the compound .

Important Questions on Chemical Kinetics
The thermal decomposition of occurs in the following steps.
Step-
Step-
Suggest the rate expression.
The reaction , obeys the following mechanism.
(i)
(ii)
Suggest the rate expression.
Given the following steps in the mechanism for a chemical reaction:
(fast)
(Slow)
(fast)
At any time is directly proportional to
(a) What is the stoichiometric equation for the reaction?
(b) Which species, if any, are catalysts in this reaction ?
(c) Which species, if any, are intermediates in this reaction?
(d) Write the rate law for the rate-determining step.
(e) Write the rate law for this reaction.
(f) What is overall order of the reaction ?
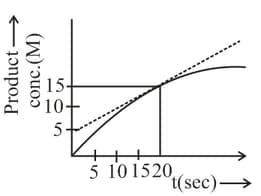
Rate of formation of product at seconds is:
In the following reaction, where, negative sign indicates rate of disappearance of the reactant. Thus, is
