An organic compound is boiled with , cooled and then treated with , a white precipitate is obtained. The compound can be
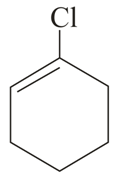
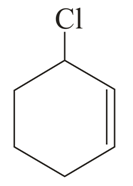
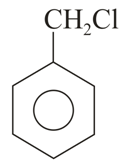

Important Questions on Purification of Organic Compounds (Qualitative and Quantitative)
Which of the organic compound will give red colour in Lassaigne's test?
An organic compound, on analysis, gave the following results :
(i) of the compound gave of carbon dioxide and of water,
(ii) of the compound give of nitrogen at NTP.
The percentage of carbon in the given compound will be?
An organic compound on analysis gave the following data:
(ii) of the compound on analysis by Duma's method gave of nitrogen gas at . Calculate the percentages of and in the organic compound if the percentage of the carbon and hydrogen is and respectively.
of an organic compound on Kjeldahl's analysis gave enough ammonia to just neutralize of .The percentage of nitrogen in the compound is:
(Atomic mass: )
In an experiment of magnesium pyrophosphate was produced from of an organic compound. The percentage of phosphorus in it is:
(Atomic mass: )
