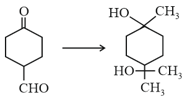
EASY
Earn 100
An organic reaction occurs through making and breaking of bonds. The breaking of bonds may occur either homolytically leading to the formation of radicals or heterolytically generating positively and negatively charged species. The neutral species (free radicals, carbenes, nitrenes, etc.) and positively charged species being electron deficient are collectively called electrophiles while neutral and negatively charged species which are electron rich are called nucleophiles. An organic reaction usually involves the attack of a reagent (radicals, positively and negatively charged species) on the substrate molecule). The substrate molecule, although as a whole electrically neutral, has centers of low and high electron density due to displacement of bonding electrons. These electron displacements occur through inductive, electromeric, resonance and hyperconjugation effects. Whereas inductive effects involve displacement -electrons, resonance effects involve transfer of - and -electrons along a conjugated system. Hyperconjugatlon effects, on the other hand, involve --conjugation. Both inductive and hyperconjugation effects can be used to explain the stability of carbocations and free radicals which follow the stabilIty order : . The stability of carbanions, however, follows opposite order.
The stability of a molecule can be judged on the basis of contribution of its resonance structures. Resonance structures have same position of nuclei and have the same number of unpaired electrons. Among resonance structures, the one which has greater number of covalent bonds, has less separation of opposite charges, a negative charge on more electronegative and a positive charge on a more electropositive atom are more stable than others.
The most stable carbanion among the following:
(a)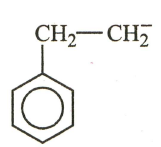

(b)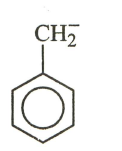

(c)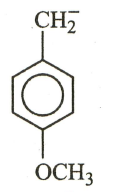

(d)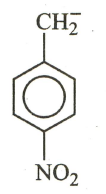

50% studentsanswered this correctly
Important Questions on Organic Chemistry- Some Basic Principles and Techniques
EASY
For the following carbocations, the correct order of stability is
I.
II.
III.
MEDIUM
In which of the following pairs is more stable than
EASY
The correct order of stability for the following alkoxides is:
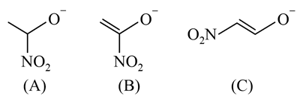
EASY
Which of the following is not a reaction intermediate?
EASY
The order of stability of the following carbocations:
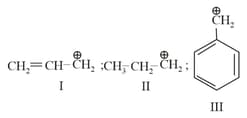
MEDIUM
Arrange the following carbanions in order of their decreasing stability
(i)
(ii)
(iii)
MEDIUM
The compound that is most difficult to protonate is:
EASY
Arrange the following free radicals in order of decreasing stability:
HARD
A solution of in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of :
MEDIUM
In which of the following compounds, the bond ionization shall give most stable carbonium ion?
MEDIUM
Which of the following carbocations is most stable?
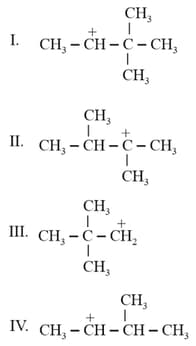
HARD
The major products and in the following reactions are
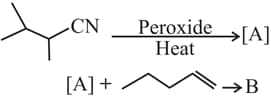
MEDIUM
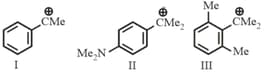
The stability order of the above carbocations is
EASY
The lower stability of ethyl anion compared to methyl anion and the higher stability of ethyl radical compared to methyl radical, respectively, are due to
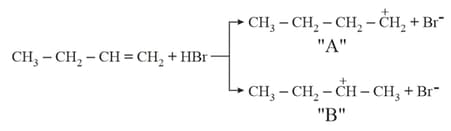
MEDIUM
Choose the correct statement regarding the formation of carbocations A and B given :-
EASY
The most stable carbocation among the following is
EASY
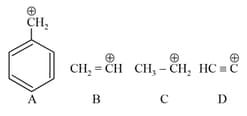
The correct order of stability of given carbocation is:
MEDIUM
The stability of carbocations

follows the order
MEDIUM
Define ambident nucleophile with an example.
MEDIUM
The correct sequence of reagents for the following conversion will be