MEDIUM
JEE Advanced
IMPORTANT
Earn 100
An unknown compound , , gives a positive test with but a negative test with Tollen's reagent. It also gives a yellow precipitate with . is:
(a)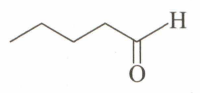

(b)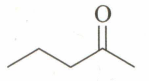

(c)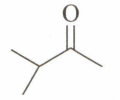

(d)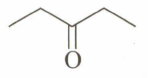

50% studentsanswered this correctly

Important Questions on Purification of Organic Compounds (Qualitative and Quantitative)
HARD
JEE Advanced
IMPORTANT
Show the scheme to separate a mixture of organic compounds containing following components; benzyl alcohol, phenol, aniline and benzoic acid.
HARD
JEE Advanced
IMPORTANT
Give a roadmap figure showing the separation of -cresol, benzoic acid and cyclohexanone.
HARD
JEE Advanced
IMPORTANT
Give a schematic diagram for the separation of the following water insoluble compounds present in a mixture.
MEDIUM
JEE Advanced
IMPORTANT
If liquid compound decomposes at its boiling point, which method(s) can you choose for its purification. It is known that the compound is stable at low pressure, steam volatile and insoluble in water.
MEDIUM
JEE Advanced
IMPORTANT
By mistake, an alcohol (boiling point ) was mixed with a hydrocarbon (boiling point ). Suggest a suitable method to separate the two compounds. Explain the reason for your choice.
HARD
JEE Advanced
IMPORTANT
Benzoic acid is an organic compound. Its crude sample can be purified by crystallisation from hot water. What characteristic differences in the properties of benzoic acid and the impurity make this process of purification suitable?
MEDIUM
JEE Advanced
IMPORTANT
In steam distillation of aniline, function of steam is:
HARD
JEE Advanced
IMPORTANT
During steam distillation of a mixture of nitrophenol and nitrophenol:
