HARD
Earn 100
An unsaturated hydrocarbon on complete hydrogenation gives 1-isopropyl - 3 - Methyl cyclohexane, after ozonolysis it gives one mole of formaldehyde, one mole of acetone and one mole of acetone and one mole of 2,4-dioxohexanedial. The possible structures of hydrocarbon are
(a)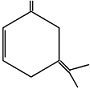

(b)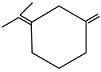

(c)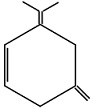

(d)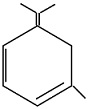

50% studentsanswered this correctly
Important Questions on Hydrocarbons
MEDIUM
Arrange the following conformational isomers of n-butane in order of their increasing potential energy:
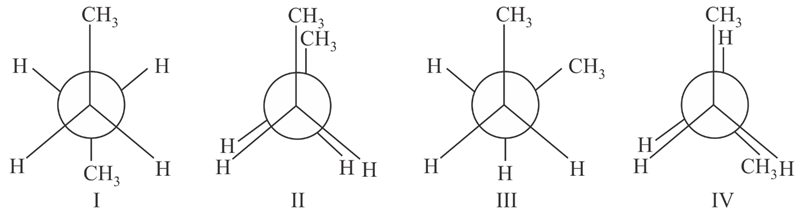
MEDIUM
HARD
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
EASY

