Analyse the electronic configuration of elements from atomic number to in the following table;
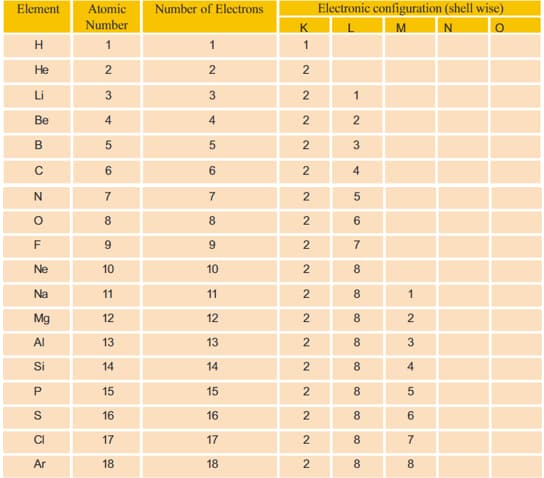
What is the maximum number of electrons that can be accommodated in L shell?


Important Questions on Structure of Atom
Complete the following table regarding the filling up of electrons in shells.
| Name of Shells | Number of Shells | Maximum number of electrons |
| K | ||
| L | ||
| M | ------- | |
| N | ---- | ------- |
Find the electronic configuration of the following atoms and draw their Bohr model.
, , .
The symbol of the aluminium atom is . Bohr model of the atom is given in the figure; analyse this and complete the table.
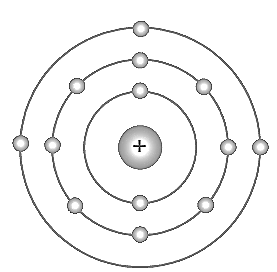
| Atomic Number | |
| Mass Number | |
| Number of Protons | |
| Number of Electrons | |
| Number of Neutrons | |
| Electronic configuration |
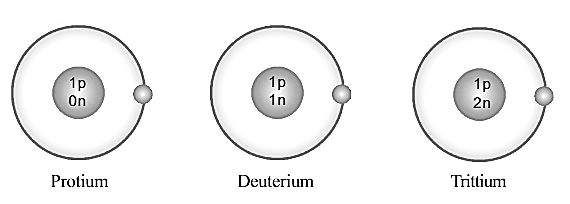
Complete the following table providing details related to these atoms.
| Name of Atom | Protium | Deuterium | Tritium |
| Number of Protons | |||
| Number of Neutrons | |||
| Number of Electrons | |||
| Atomic Number | |||
| Mass Number |
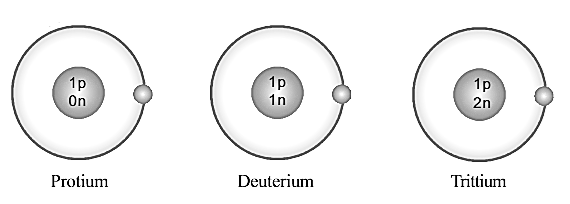
Which is the particle that differs in its number in these atoms?
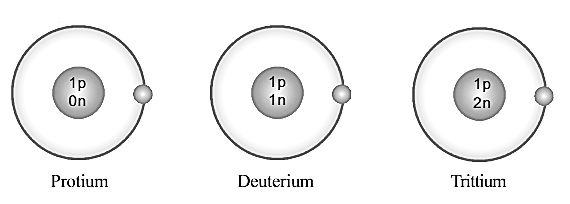
What interference can you arrive at when the mass number and an atomic number of these elements are examined?
Symbols of certain elements are given in the table. Complete the table writing there atomic number, mass number, number of protons, electrons and neutron.
| Symbol | Atomic Number | Mass Number | Protons | Electrons | Neutrons |
The atomic number of an atom Z= , mass number A= .
Find the number of protons, electrons and neutrons in the atom.
