HARD
JEE Main/Advance
IMPORTANT
Earn 100
Aniline behaves as a weak base. When solution sample of aniline was mixed with solution of the of the resulting solution was Then:
(a) of solution of anilinium chloride is
(b)of original solution of aniline is
(c)Upon adding the same aniline sample to the above mixture, of resulting solution becomes
(d)Upon adding the same sample to the above mixture, of resulting solution becomes
50% studentsanswered this correctly

Important Questions on Equilibrium
HARD
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
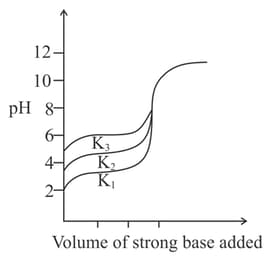
Titration curves for solutions of three weak acids and with ionization constants and respectively are plotted as shown in the figure. Which of the following is/are true?
MEDIUM
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
In above question, If order of precipitation will be:
HARD
JEE Main/Advance
IMPORTANT
EASY
JEE Main/Advance
IMPORTANT
MEDIUM
JEE Main/Advance
IMPORTANT
HARD
JEE Main/Advance
IMPORTANT
Consider the coordination compound, [Co(NH3)6]Cl3. In the formation of this complex, the species which acts as the Lewis acid is :
