MEDIUM
Earn 100
Answer the following in one sentence:
How are basic buffer solutions prepared?
Important Questions on Ionic Equilibria
MEDIUM
HARD
MEDIUM
MEDIUM
Which of the following choices is correct for a mixture of and
EASY
MEDIUM
HARD
EASY
HARD
MEDIUM
MEDIUM
The stoichiometry and solubility product of a salt with the solubility curve given below is, respectively:
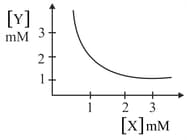
EASY
MEDIUM
MEDIUM
EASY
EASY
EASY
If the solubility product of is , then the solubility of in pure water is _______
[Assuming that neither kind of ion reacts with water]
MEDIUM
MEDIUM
[Solubility product for ]
MEDIUM

