EASY
Earn 100
Aqueous acid solutions conduct electricity because they are identified as . (electrolyte/non-electrolyte)
100% studentsanswered this correctly
Important Questions on Acids, Bases and Salts
EASY
MEDIUM
MEDIUM
Four solutions A, B, C, and D have pH 2, 6. 7, and 13 respectively:
Which solution will have the highest number of hydronium ions?
MEDIUM
EASY
MEDIUM
The bulb is not glowing in this experimental setup. Because:
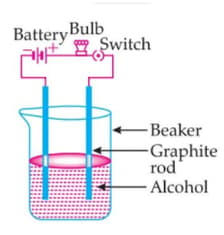
MEDIUM
What is an alkali? Do basic solutions also have (aq) ions? If yes, then why are these basic?
EASY
Which of the following is a strong acid?
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
HARD
MEDIUM
HARD
HARD

