MEDIUM
Earn 100
Aqueous solutions of two compounds and are prepared in two different beakers. If the electronegativity of then the nature of the two solutions will be respectively.
(a)acidic, basic
(b)acidic, acidic
(c)basic, acidic
(d)basic, basic
66.67% studentsanswered this correctly
Important Questions on Classification of Elements and Periodicity in Properties
MEDIUM
Sodium metal on dissolution in liquid ammonia gives a deep blue solution due to the formation of:
EASY
The correct order of electronegativity for given elements is
EASY
The metal that forms nitride by reacting directly with of air is:
MEDIUM
The electronegativities of and are in the order -
EASY
Which of the following indicates the correct variation in eletronegativities?
EASY
The hottest region of Bunsen flame shown in the figure below is:
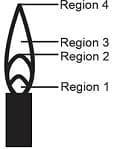
MEDIUM
A metal on combustion in excess air forms . upon hydrolysis with water yields and along with another product. The metal is:
EASY
On combustion of and in excess of air, the major oxides formed, respectively, are:
EASY
The most electronegative element from the following elements is:
EASY
Arrange as directed:
belonging to Group 16 of the long periodic table according to increasing order of electronegativity.
(The atomic numbers have been given within first brackets after the symbols of the elements)
EASY
The main oxides formed on combustion of Li, Na and K in excess of air are respectively:
EASY
The electronegativity of the given elements increases in the order
MEDIUM
The correct order of hydration enthalpies of alkali metal ions is:
MEDIUM
Identify the weakest oxidizing agent among the following
EASY
The metal mainly used in devising photoelectric cells is :
EASY
The oxide of potassium that does not exist is
HARD
Which of the following liberates upon hydrolysis?
EASY
Most electronegativity element in the periodic table
MEDIUM
Given that ionization potential and electron gain enthalpy of chlorine are and respectively. The electronegativity of chlorine on Mulliken scale, approximately equals to
EASY
Identify the correct order of electronegativity for and .

