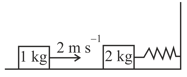
MEDIUM
Earn 100
Area of piston is . When heat is supplied to the gas it expands and displaces piston by , where . Natural length of springs is . Spring constant . The pressure of gas in final situation is – (considering equilibrium)
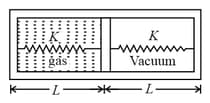

(a)
(b)
(c)
(d)
50% studentsanswered this correctly
Important Questions on Kinetic Theory of Gases
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
MEDIUM
EASY
EASY
Some smoke is trapped in a small glass container and is viewed through a microscope. A number of very small smoke particles are seen in continuous random motion as a result of their bombardment by air molecules. If the mass of the smoke particle is about times higher than that of an air molecule the average speed of a smoke particle is
MEDIUM
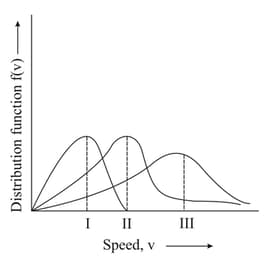
HARD
MEDIUM
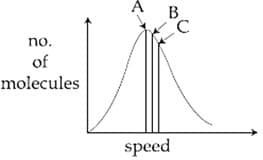
Root mean square speed most proable speed Average speed
EASY
EASY
MEDIUM
MEDIUM
EASY
Which of the following curves represent the variation of coefficient of volume expansion of an ideal gas at constant pressure?
EASY
MEDIUM