MEDIUM
NEET
IMPORTANT
Earn 100
Argon gas is at initial temperature . If gas is expanded adiabatically 8 times to its initial volume then find final temperature of Argon gas?
(a)
(b)
(c)
(d)
100% studentsanswered this correctly

Important Questions on Thermodynamics
MEDIUM
NEET
IMPORTANT
A sample of of water at and normal pressure requires of heat energy to convert to steam at . If the volume of the steam produced is the change in internal energy of the sample, is
MEDIUM
NEET
IMPORTANT
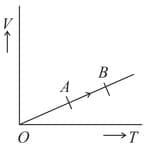
The volume () of a monatomic gas varies with its temperature (), as shown in the graph. The ratio of work done by the gas, to the heat absorbed by it, when it undergoes a change from state to state , is
EASY
NEET
IMPORTANT
The efficiency of a heat engine working between the freezing point and boiling point of water is
MEDIUM
NEET
IMPORTANT
Which of the following properties of a thermodynamic system are extensive?
MEDIUM
NEET
IMPORTANT
pressure is heated to convert it to vapour. Find increase in internal energy if latent heat of vaporization of water
MEDIUM
NEET
IMPORTANT
Which of the following is the correct intensive property or a thermodynamic system?
MEDIUM
NEET
IMPORTANT
Work done in expanding one mole of an ideal gas isothermally from to is equal to the work done in expanding three moles of an ideal gas from at same temperature. Find .
MEDIUM
NEET
IMPORTANT
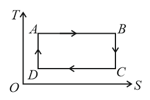
Consider the given temperature entropy diagram. Which of the following processes represent increase in volume ?