EASY
10th Chhattisgarh Board
IMPORTANT
Earn 100
Arrange the elements in the table in increasing order of their atomic weights. Arrange the elements in the table in increasing order of their atomic numbers. Did you get the same order of elements in both the cases?
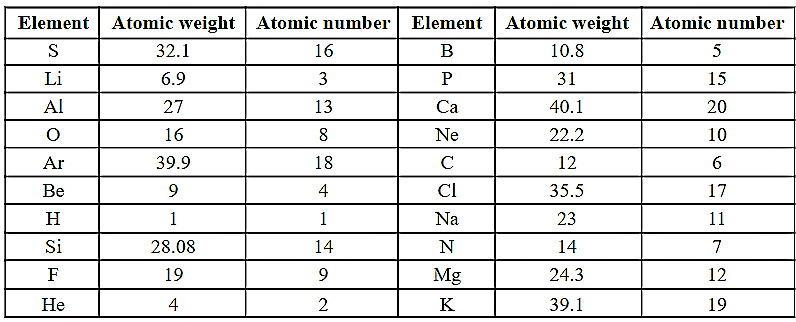


Important Questions on Periodic Classification of Elements
MEDIUM
10th Chhattisgarh Board
IMPORTANT
Write the electronic configurations of the given elements.
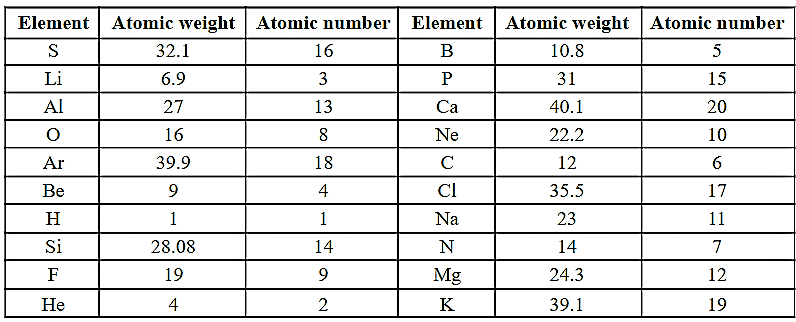
HARD
10th Chhattisgarh Board
IMPORTANT
Can we group the elements based on their electronic configuration?
EASY
10th Chhattisgarh Board
IMPORTANT
Look at the given table and answer:
Which element will lie in the middle of group ‘A’?
| Group A | Element | |||
| Atomic mass | ||||
| Group B | Element | |||
| Atomic mass | ||||
| Group C | Element | |||
| Atomic mass |
EASY
10th Chhattisgarh Board
IMPORTANT
Look at the given table and answer:
What will be the atomic weight of the middle element in group ‘B’?
| Group A | Element | |||
| Atomic mass | ||||
| Group B | Element | |||
| Atomic mass | ||||
| Group C | Element | |||
| Atomic mass |
EASY
10th Chhattisgarh Board
IMPORTANT
Look at the table and answer:
What will be the atomic weight of the element in group ‘C’?
| Group A | Element | |||
| Atomic mass | ||||
| Group B | Element | |||
| Atomic mass | ||||
| Group C | Element | |||
| Atomic mass |
MEDIUM
10th Chhattisgarh Board
IMPORTANT
EASY
10th Chhattisgarh Board
IMPORTANT
Look at the table and identify the elements that sodium will resemble.
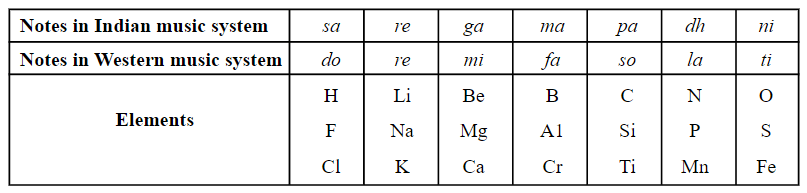
EASY
10th Chhattisgarh Board
IMPORTANT
What was the basis of the classification of elements as done by Lothar Meyer?
