MEDIUM
MYP:4-5
IMPORTANT
Earn 100
As a solution of barium hydroxide is mixed with an aqueous solution of sulphuric acid, a white precipitate forms and the electrical conductivity decreases markedly. Write down word and symbol equations for the reaction that occurred.

Important Questions on What are the Impacts of Chemical Industry?
MEDIUM
MYP:4-5
IMPORTANT
As a solution of barium hydroxide is mixed with an aqueous solution of sulphuric acid, a white precipitate forms and the electrical conductivity decreases markedly. Suggest an explanation for the change in conductivity.
MEDIUM
MYP:4-5
IMPORTANT
When solid sodium ethanoate is added to an aqueous solution of hydrogen fluoride , a reaction occurs in which the weak acid loses hydrogen ions . Write down word and symbol equation for the reaction that occurred.
MEDIUM
MYP:4-5
IMPORTANT
When solid sodium ethanoate is added to an aqueous solution of hydrogen fluoride , a reaction occurs in which the weak acid loses hydrogen ions . Suggest the identity of the ion that is competing with the fluoride ion, for the proton.
MEDIUM
MYP:4-5
IMPORTANT
Suggest why, although the reaction of sodium with dilute hydrochloric acid is almost explosive, the reaction between sodium and ethanoic acid (at the same concentration and temperature) is only a little faster than that between sodium and water.
MEDIUM
MYP:4-5
IMPORTANT
Table lists the strength of various acids, familiar and unfamiliar, and their dissociation in water. Analyse the information and make a scientifically supported judgement about acid that have more than one ionisable hydrogen atom in their molecule or ions.
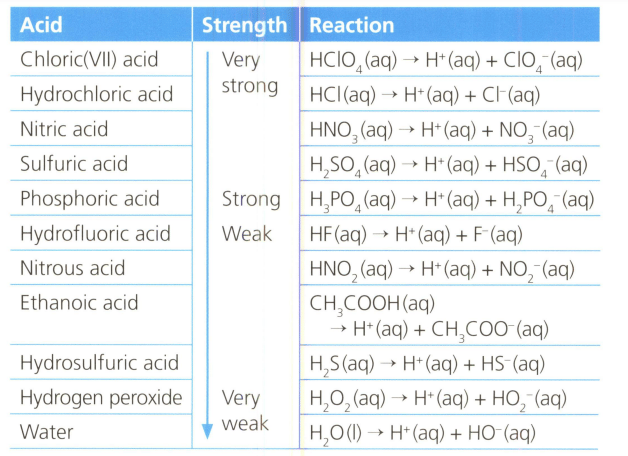
MEDIUM
MYP:4-5
IMPORTANT
Use the Bronsted-Lowry theory to explain (with examples) why water is amphoteric in acid-base reaction.
MEDIUM
MYP:4-5
IMPORTANT
Explain the distinction between strong and weak versus concentrated and dilute acids. Include diagrams to represent these concepts.
HARD
MYP:4-5
IMPORTANT
The definitions below represent some of the emerging ideas by scientists reflecting on the chemistry of acids and bases.
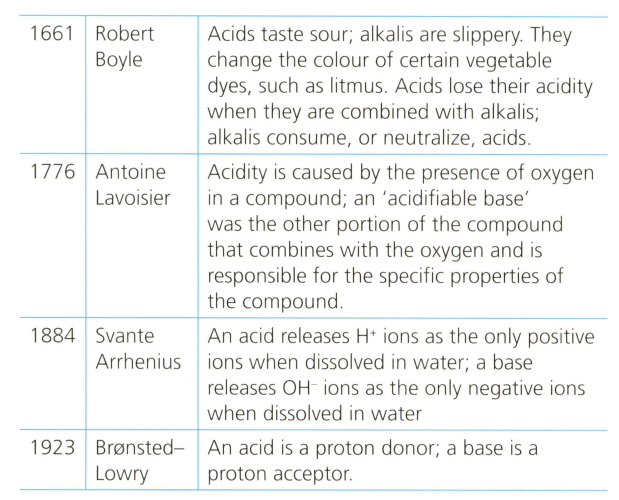
Analyse and evaluate these definitions and make scientifically supported judgement about whether any parts of these definitions are satisfactory in the light of your knowledge.
