MEDIUM
12th ICSE
IMPORTANT
Earn 100
As per Bohr model, the minimum energy (in ) required to remove an electron from the ground state of (doubly-ionised atom) is:
(a)
(b)
(c)
(d)
50% studentsanswered this correctly

Important Questions on Atom, Origin of Spectra : Bohr's Theory of Hydrogen Atom
MEDIUM
12th ICSE
IMPORTANT
If elements with principal quantum number did not exist in nature, the number of possible elements would be:
MEDIUM
12th ICSE
IMPORTANT
The transition from to in a hydrogen like atom result in ultravoilet radiation. Infrared radiation will be obtained in the transition from:
MEDIUM
12th ICSE
IMPORTANT
Hydrogen Deuterium , singly ionised Helium and double ionised lithium all have one electron around the nucleus. Consider an electron transition from to . If the wave lengths of emitted radiation are respectively then approximately which one of the following is correct?
MEDIUM
12th ICSE
IMPORTANT
Hydrogen atom in ground state is excited by a monochromatic radiation of . The number of spectral lines in the resulting spectrum emitted will be
EASY
12th ICSE
IMPORTANT
Energy required for the electron excitation in from the first to the third Bohr orbit is?
EASY
12th ICSE
IMPORTANT
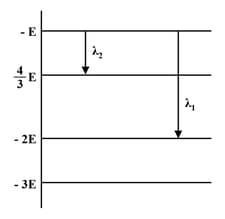
Some energy levels of a molecule are shown in the figure. The ratio of the wavelengths is given by :
HARD
12th ICSE
IMPORTANT
The radiation corresponding to transition of hydrogen atom falls on a metal surface to produce photoelectrons. These electrons are made to enter a magnetic field of . If the radius of the largest circular path followed by these electrons is 10.0mm, the work function of the metal is close to
EASY
12th ICSE
IMPORTANT
The ratio of wavelength of the last line of Balmer series and the last line Lyman series is:
