EASY
Diploma
IMPORTANT
Earn 100
Aspirin and ibuprofen are painkillers. The structures of Aspirin and ibuprofen are shown in figure.
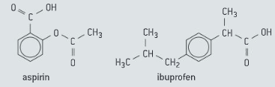
State the number of peaks in the NMR spectrum of aspirin (ignore the peaks due to the hydrogen atoms on the benzene ring and the reference sample).


Important Questions on Medicinal Chemistry
MEDIUM
Diploma
IMPORTANT
Describe the splitting pattern for each of the peaks in NMR spectrum of aspirin.
EASY
Diploma
IMPORTANT
State how the infrared spectra of aspirin and ibuprofen will differ in the region .
EASY
Diploma
IMPORTANT
Pharmacological properties of drugs depend upon their polarities. The partition coefficient of a certain drug between cellular tissues and blood plasma is . Calculate the concentration, in , this drug in tissues if its concentration in the blood plasma is maintained at by continuous injection.
EASY
Diploma
IMPORTANT
Extraction is an important technique in medicinal chemistry. Outline how a mixture of two organic compounds with different polarities can be separated by extraction.
EASY
Diploma
IMPORTANT
The partition of a pharmaceutical drug () between water and an organic solvent can be represented by the equation, . Deduce the equation for the partition coefficient of .
EASY
Diploma
IMPORTANT
An aqueous solution with was extracted with an equal volume of Octan--ol. After the extraction, the concentration of in the aqueous phase decrease to . Calculate the concentration of in the organic phase and the value for this drug.
EASY
Diploma
IMPORTANT
Anabolic steroids are used by some athletes as performance-enhancing substances. Explain how steroids and other illegal drugs can be detected in the human body chromatography and mass spectrometry?
MEDIUM
Diploma
IMPORTANT
Ethanol is sufficiently volatile to pass into the lungs from the bloodstream. The roadside breathalyzer uses potassium dichromate (), which reacts with ethanol present in the breath. Deduce the oxidation and reduction half-equations that occur in the breathalyzer.
