EASY
Earn 100
Assertion: .The rms speed of helium gas is equal to rms speed of hydrogen gas when temperature of helium gas is twice that of hydrogen gas.
Reason: rms speed of a gas depends only on the temperature of gas.
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not correct explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
50% studentsanswered this correctly
Important Questions on Kinetic Theory of Gases
HARD
HARD
Consider an ideal gas confined in an isolated closed chamber. As the gas undergoes an adiabatic expansion, the average time of collision between molecules increases as , where is the volume of the gas. The value of is:
MEDIUM
MEDIUM
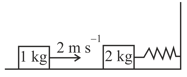
EASY
MEDIUM
MEDIUM
EASY
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
Boltzmann Constant
Avogadro number
Radius of Earth:
Gravitational acceleration on Earth
EASY
EASY
EASY
EASY
EASY
EASY
EASY

