Assertion: First law of thermodynamics can be applied only for an ideal or real gas system.
Reason: First law of thermodynamics is nothing but law of conservation of energy.

Important Questions on The First Law of Thermodynamics
Assertion: There are two processes : Process 1 is constant and process is constant. In both the processes volume of gas is increased from to . Initial coordinates of the gas were the same. Then, more work is done by the gas in process .
Reason: In second process pressure drops more rapidly with increase in volume.
Assertion: Any process taking place in the atmosphere is considered as an isobaric process.
Reason: Work done by the system in that case is . Where, is atmospheric pressure.
Assertion: During melting of ice work done by surrounding on (ice + water) system is positive.
Reason: Volume of the given system decreases on melting of ice.
Assertion: Molar heat capacity cannot be defined for an isothermal process.
Reason: In isothermal process versus graph is a dot.
Assertion: If the initial and the final volumes are equal, then work done by the gas is zero.
Reason In an isochoric process the initial and the final volumes are equal and work done by gas is zero.
Assertion: In adiabatic expansion the product of and always decreases.
Reason: In adiabatic expansion process, work is done by the gas at the cost of internal energy of gas.
Assertion: In isobaric process is equal to of the gas.
Reason: For monoatomic gas ratio in the isobaric process is equal to .
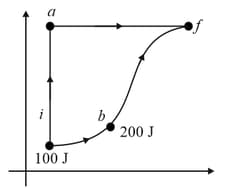
A thermodynamic system is taken from an initial state with internal energy to the final state along two different paths and as schematically shown in the figure. The work done by the system along the paths and are and respectively. The heat supplied to the system along the path and are and respectively. If the internal energy of the system in the state is and , then the ratio is