MEDIUM
Earn 100
Assertion: For an ideal gas, at constant temperature, the product of the pressure and volume is constant.
Reason: The mean square speed of the molecules is , = Boltzman's constant, m = mass of molecule.
(a)If both Assertion and Reason are true and the Reason is correct explanation of the Assertion.
(b)If both Assertion and Reason are true but Reason is not correct explanation of the Assertion.
(c)If Assertion is true but the Reason is false.
(d)If Assertion is false but Reason is true.
50% studentsanswered this correctly
Important Questions on Heat and Thermodynamics
MEDIUM
HARD
EASY
EASY
HARD
Match the following:
| List-1 | List-2 |
| A) Global warming B) Eutrophication C) Biomagnification D) Ozone depletion |
I) Mercury II) UV-B rays III) Methane IV) Algal blooms V) Terror of Bengal |
The correct match is:
MEDIUM
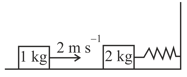
EASY
MEDIUM
Boltzmann Constant
Avogadro number
Radius of Earth:
Gravitational acceleration on Earth
EASY
HARD
EASY
MEDIUM
EASY
MEDIUM
MEDIUM
EASY
MEDIUM
MEDIUM
MEDIUM
| LIST-I | LIST-II | ||
| A | Radioactive wastes | I | Global warming |
| B | Greenhouse effect | II | Snow blindness |
| C | UV — B rays | III | Biomagnification |
| D | DDT | IV | Mutations |
| V | Eutrophication |
The correct match is
EASY

